Clinical Trials
Each year in Scotland more than 1500 clinical research studies take place, involving over 30,000 patients. During the COVID-19 pandemic over 60,000 patients were involved in vital research to develop treatments and vaccines. Taking part in research can offer many benefits. It may provide access to new treatments or help participants learn more about their condition. The close monitoring of participants by healthcare professionals during a research study can also help to improve outcomes
What Are Clinical Trials
Clinical trials can help show whether new approaches to cancer treatment work more effectively than current methods and test whether they are safe when compared to the current standard of care. All new anti-cancer drugs need to go through rigorous testing before they can become routinely available to patients.
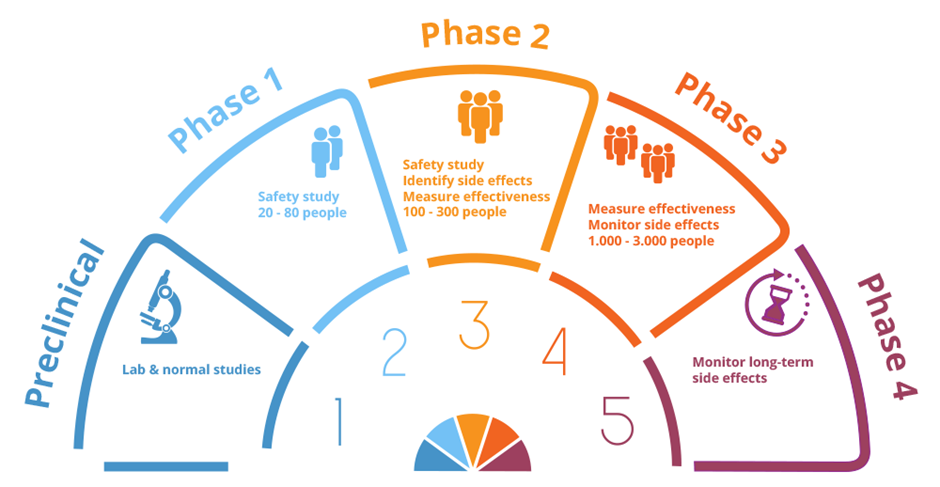
After initial laboratory testing, there are three main phases of clinical trials in developing new drugs. Each stage has a different aim.
Phase 1: Early phase trials usually involve small numbers of patients and mainly aim to establish a safe dose for the drug and identify possible side effects. Some phase I studies now also have ‘expansion cohorts’, studying small groups of patients in cancers thought most likely to benefit from the new agent(s)
Phase 2: Phase 2 studies usually involve approximately 100-300 participants with the same type of cancer and look at the different ways of giving the drug(s) and whether they are effective
Phase 3: Phase 3 trials usually involve large numbers of patients. They aim to test a new treatment against the best available standard treatment, to see which is more effective, and also to monitor possible side-effects.
Why Are Clinical Trials Important
Clinical trials help doctors and researchers learn more about cancer and how to treat it more effectively. Research improves our understanding of how cancer behaves and allows us to test new methods of treatment. It is only through properly conducted research that new treatments can be shown to be better and become established as standard practice.
Patient safety during any clinical trial is always the number one priority, so learning how best to treat cancer through clinical trials is an important part of patient care in cancer medicine, from diagnosis to treatment. Clinical trials can often offer patients access to promising new drugs at the earliest opportunity, as unfortunately it does take time for drugs to become fully assessed and approved.
Volunteers for clinical research often want to take part because their involvement helps to shape the future of treatment for others, as well as for themselves.
How Do Patients Participate in Trials?
Patients who potentially meet the criteria for a particular trial are first given a Patient Information Sheet (PIS) by their oncologist. The PIS outlines the purpose of the study, who is running the study, what is involved in being a participant, how long the study will last and other key pieces of information for patients to read about. Each study requires participants to give their ‘informed consent’, before participating in a study. This means that each potential participant has sufficient time to read, discuss and understand the purpose, risks, and potential outcomes of the study.
If a patient agrees to take part, they will be asked to sign a study consent form. The form is an acknowledgement of the patient’s own willingness to take part in the study, which can also be withdrawn at any time. Patients may withdraw from a trial whenever they wish during their participation for any reason. If a patient does withdraw from a trial, they are still entitled to receive the care that is currently the best option for them.
Clinical trial eligibility
Most studies have a strict list of criteria that any potential participants need to meet, before being able to enrol on the study. This is both for patient safety, to ensure trial participants are not at increased risk of serious side-effects, and also to ensure the study is being undertaken using the planned patient population. For example, it is often important that all patients in the trial are at the same stage of their cancer journey, as cancers can respond differently if they have already been exposed to certain previous drugs.
Before commencing participation, most studies require patients to undergo ‘screening’ tests, to check that the trial is suitable and safe for them. This could include blood tests, scans, other minor medical tests such as eye or breathing tests, or questionnaires. Sometimes, despite being hopeful of enrolling on a clinical trial, a patient can unfortunately not meet the required criteria if it is not safe for them to do so.
Randomisation
Some studies involve ‘randomisation’ between different treatments, where a computer will decide which treatment group a participant is allocated. This is very important, as, when it comes to comparing the results, the different treatment groups are as evenly matched as possible, so that the only real difference between the two groups is the new treatment. The study doctor usually has no influence on which treatment group a patient is allocated to.
Placebos in cancer trials
Many anti-cancer drug trials do not involve the use of placebos. If a study is being offered to you which does involve a placebo (a non-active drug), this will be very clearly stated in the trial information, and the use of placebo will have been very closely considered by the research ethics committee (REC). Placebos are only usually ever used on their own in cancer studies if there is no other standard treatment recommended in that setting. More commonly, placebos in cancer trials are used as an extra treatment, to be given in addition to other anti-cancer therapy. Sometimes, neither the patient nor their doctor know which treatment group they have been allocated to; this is called a ‘double-blind’ study.
Trial treatment
During the treatment phase of the study, patients will be provided with clear instructions and information regarding the treatment from consultants and research nurses. This could include attending regularly scheduled appointments, taking prescribed medicines, and completing paperwork such as a side-effect diary. Patient safety and wellbeing is monitored closely while participating in the study and may require follow-up tests and paperwork to be completed on a regular basis.
Once patients complete the treatment phase of the study, there may still be tests and paperwork to complete on a less regular basis. There may also be follow up visits required to ensure each patient’s long-term safety and wellbeing. The patient will carry on receiving their best treatment option once study participation is completed. In some studies, patients can receive financial compensation for their time and travel, as some studies do involve more intensive follow-up than would be usual
What are the potential benefits of taking part in a clinical trial
There can be a number of benefits of participating in a clinical trial. Some studies can offer earlier access to new drugs or improved technology/techniques, although it is important to be aware that not all new drugs do end up being proven to be better. Many patients do report they receive more personal, closer support or side-effect management, with continuity of care from research nurses whom they get to know well. Some patients are also keen to enter research studies as there is longer or more comprehensive follow-up that would otherwise be offered in routine clinical care, although others can be put off by the requirement for extra tests or visits.
There are also various benefits for cancer centres by participating in clinical research; it can improve the quality of care offered in the centre as a whole, as there are often quality assurance processes required for clinical trials. It also allows healthcare professionals to gain experience with new drugs or techniques at the earliest opportunity, in a carefully managed way. Being involved in research can also help with recruitment or retention of enthusiastic staff, as many are keen to be involved.
The most important thing for any patient considering potential research study participation is to be fully aware of what is expected and discussing it with their doctor in detail. It is also important to know that withdrawal from any clinical trial is always an option.
Patient and Public Involvement
You may be interested in having a say in how research is carried out.
All research is improved when patients and the public are involved from the start. Patient and Public Involvement (or PPI) means that people can use their own experiences to shape the research of the future. Talking to the people who use and care about the NHS services leads to a greater understanding of the needs of our communities and helps our research to be more relevant and of greater benefit.
This may involve:
- sharing knowledge and insights about your condition with researchers
- helping researchers to explain their research in understandable ways
- improving patient information leaflets
- helping design research
The Edinburgh Cancer Centre PPI group has been set up to ensure that people affected by cancer have a strong platform to shape cancer research. The group meets with researchers online four times per year and members share their personal experiences of cancer to help inform our trials. Members of the group have either had a diagnosis of cancer themselves or have a close family member with a diagnosis.
If you are interested in finding out more about PPI at the Edinburgh Cancer Centre please contact:
Lois Eddie (CRUK Senior Research Nurse) at lois.eddie@nhs.scot
Jenny Sharma (PPI Manager, CRUK Scotland Centre) at jenny.sharma@ed.ac.uk
